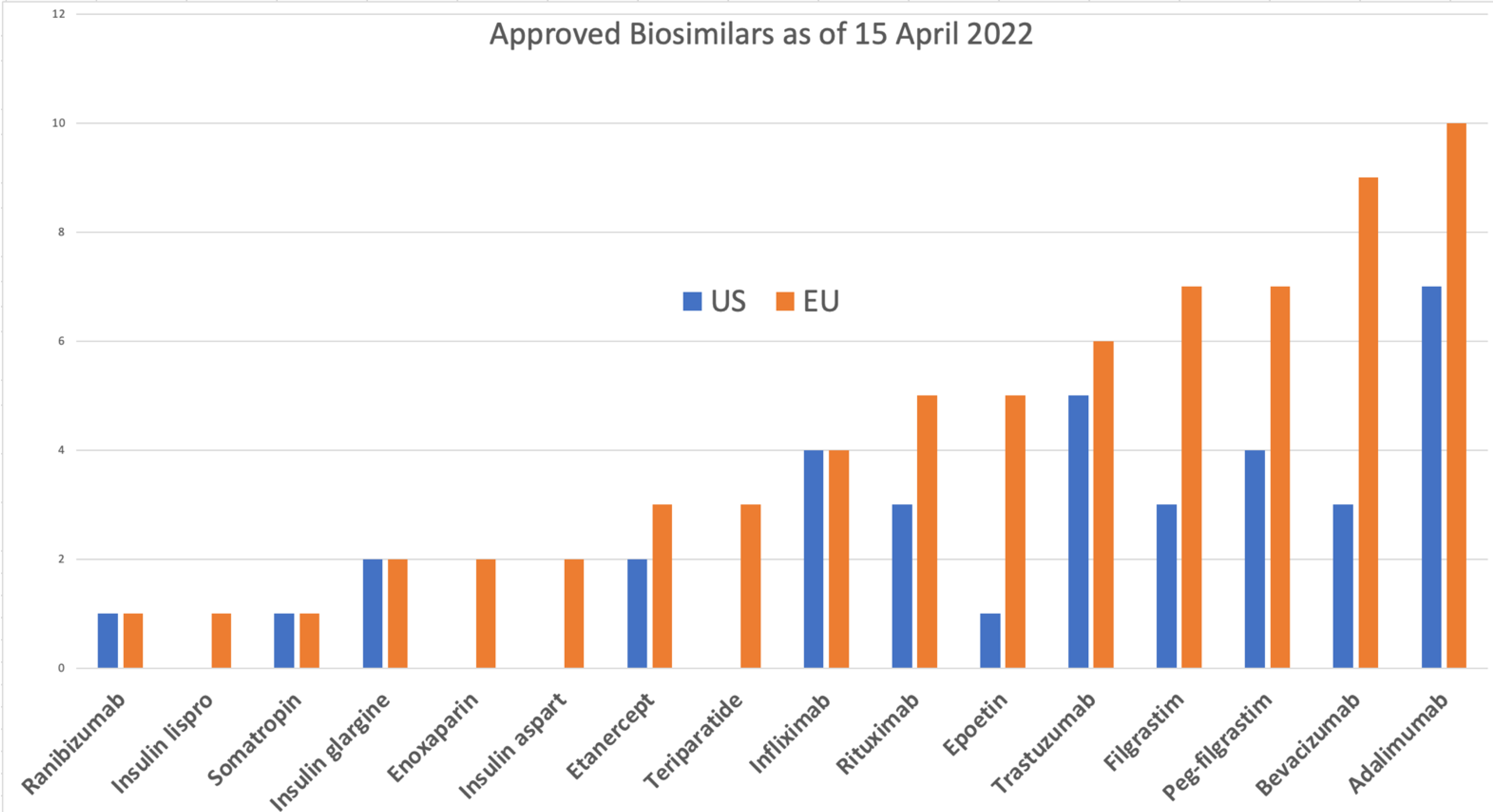
Inês Alves - Member of the Committee for Orphan Medicinal Products - COMP - European Medicines Agency | LinkedIn
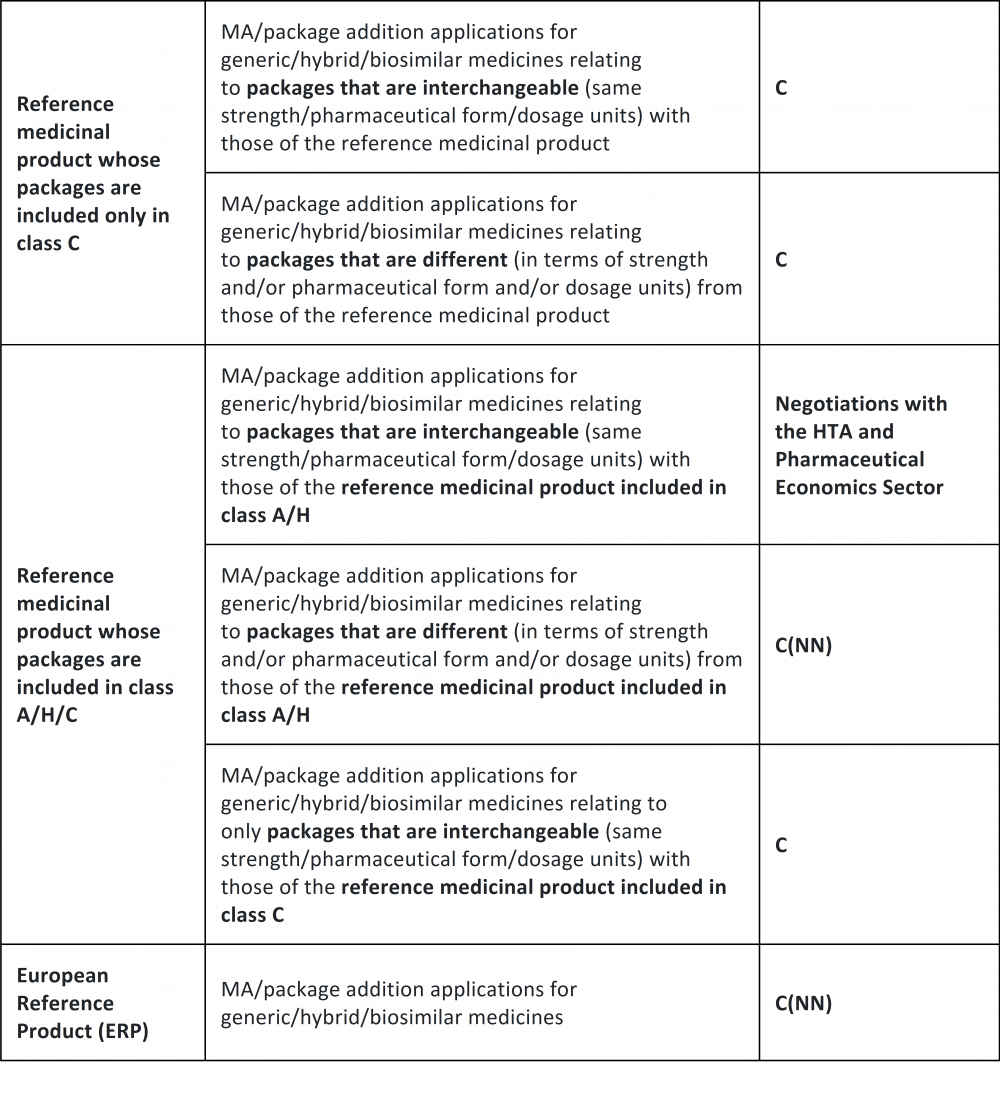
The Italian Medicines Agency provides additional information on the new simplified classification procedure for generics and biosimilars - Portolano Cavallo
Marketing authorisations which are recommended for maintenance and marketing authorisation applications for which bioequivalence

Book 4C: 2023 Good Manufacturing Practice in the European Union, Refer – Clinical Research Resources, LLC

Marketing authorization and licensing of medicinal products in EU: Regulatory aspects - ScienceDirect
The european substance reference system (EU - SRS) release strategy widely supported by the community - UNICOM