Investigational Medicinal Product Dossier | IMPD | Non Clinical Drug Development | DRA | PharmaWins - YouTube
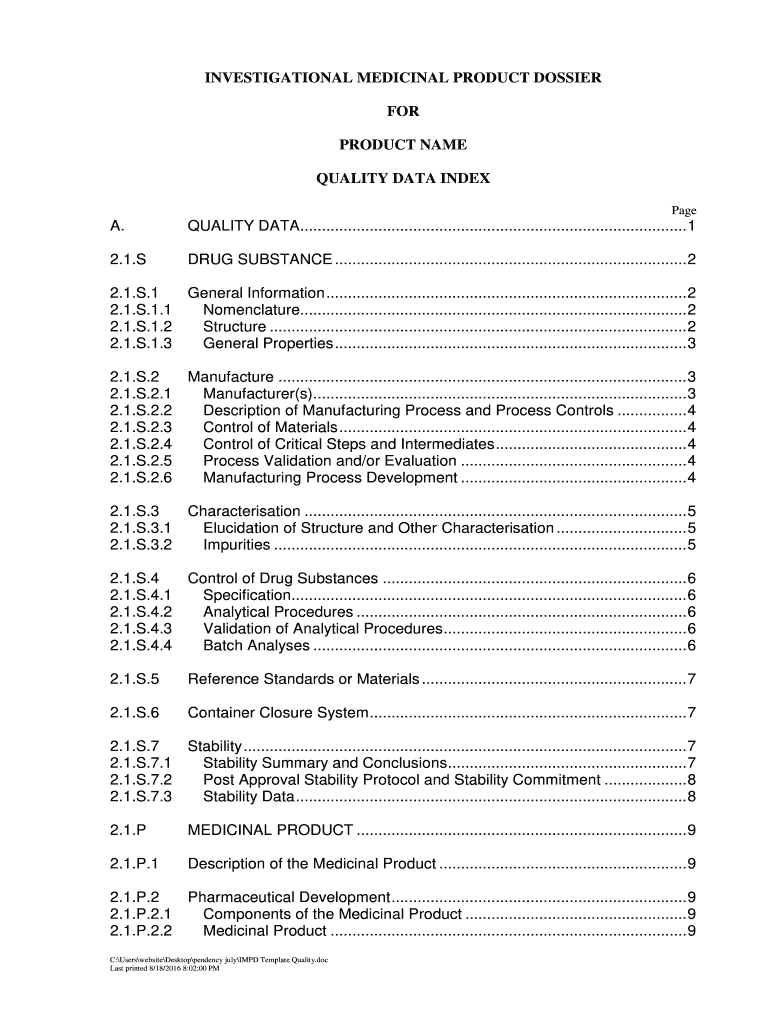
MANUAL TO WRITE AN INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER FOR A (SOMATIC) CELL THERAPY MEDICINAL PRODUCT

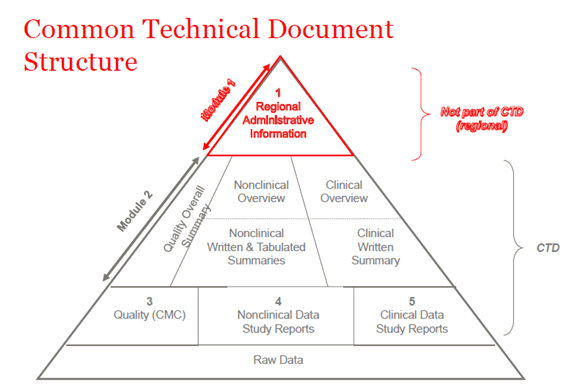
Proposed clinical trial application dossier. AMPs auxiliary medicinal... | Download Scientific Diagram